In case you missed it, on February 24th, 2012 there was another birth control pill recall. This time from Glenmark Generics Inc., Norgestimate and Ethinyl Estradiol Tablets USP, 0.18 mg/0.035 mg, 0.215 mg/0.035 mg, 0.25 mg/0.035 mg.
The tablets were distributed to wholesalers and pharmacies nationwide between September 21, 2011 and December 30, 2011 which means you very well could have some in your possession.
“The recall is being implemented because of a packaging error, where select blisters were rotated 180 degrees within the card, reversing the weekly tablet orientation and making the lot number and expiry date visible only on the outer pouch. Any blister for which the lot number and expiry date is not visible is subject to recall.
As a result of this packaging error, the daily regimen for these oral contraceptives may be incorrect and could leave women without adequate contraception, and at risk for unintended pregnancy. These packaging defects do not pose any immediate health risks. However, consumers exposed to affected packaging should begin using a non-hormonal form of contraception immediately.”
Visit the following link to read the full press release, check the lot numbers & expiration dates of the affected products, and find out who to contact. http://www.fda.gov/Safety/Recalls/
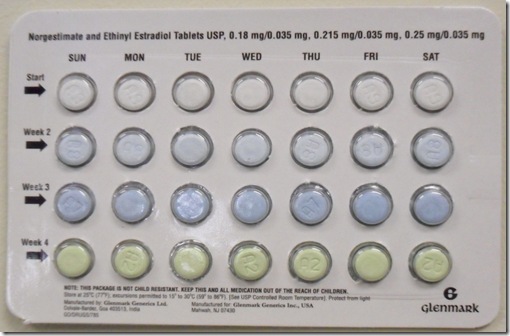
Photo of affected product for reference. © FDA






